
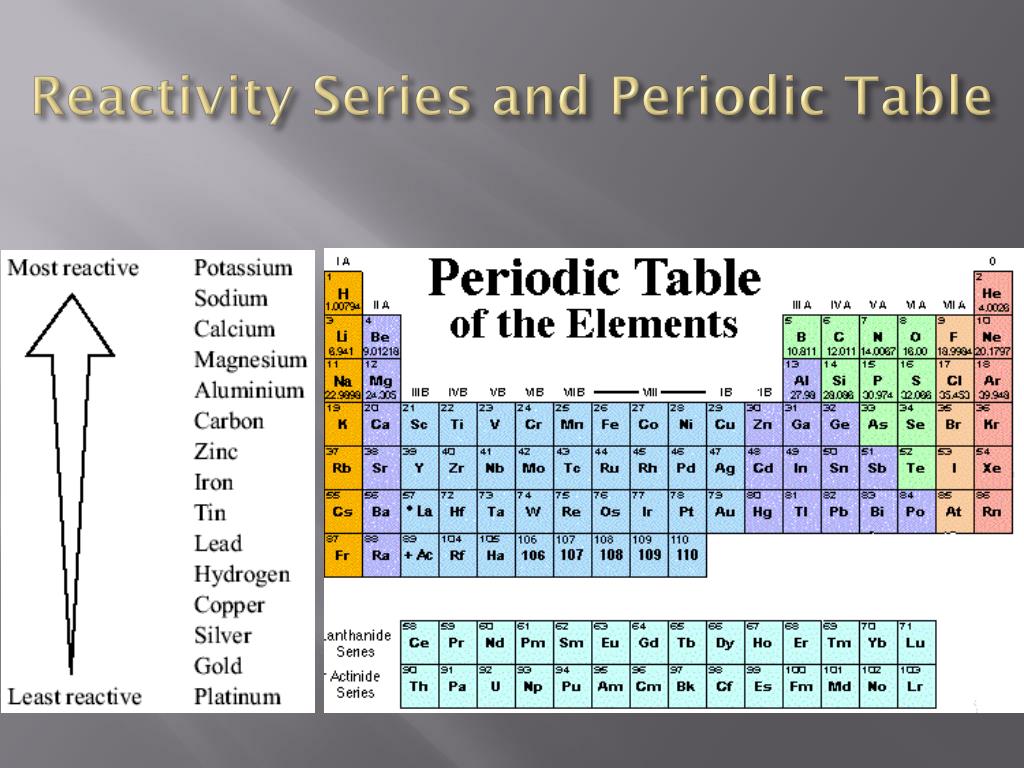
Longman Pocket Study Guide 'O' Level Science-Chemistry by Lim Eng Wah Chapter 8 pg 190.The table below is an activity series of most common metals and of the halogens. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. Pearson Education South Asia Pte Ltd 2005. The activity series is a list of elements in decreasing order of their reactivity. Science in Focus, Chemistry for GCE 'O' Level by J G R Briggs Chapter 11 pg 172.Only a metal higher in the reactivity series will displace another.

Therefore the elemental metal will 'displace' the ionic metal over time, thus the two swap places.

When a metal in elemental form is placed in a solution of a metal salt it may be, overall, more energetically feasible for this "elemental metal" to exist as an ion and the "ionic metal" to exist as the element. However it is defined by the nature of the metals in single displacement reactions. The reactivity series determines qualitatively characteristics such as the reactions with water, air and acids as demonstrated above.
does not readily give up electrons in reactions to form positive ions. does not react vigorously and quickly with chemicals Series Report Historical News Release Tables Maps Calculators Public Data API. readily gives up electrons in reactions to form positive ionsĪ metal 'low down' in the reactivity series:. reacts vigorously and quickly with chemicals. Higher education and standard level are required to study more metals as shown above.Ī metal 'high up' in the reactivity series: The simplified version that is taught in the GCSE and GCE 'O' Level chemistry course, as the basic, are listed below. This series has metal atoms arranged from higher reactivity to lower reactivity. See Table of standard electrode potentials). The activity series of metals describes the relative reactivity of metals against the standard hydrogen. The reactivity series has applications in electrochemistry, where two dissimilar metals are chosen as electrodes of a battery (though the above table is not exact for this purpose. Metals that can replace hydrogen within acids but not water are listed in the middle of the activity series, for example zinc replaces hydrogen in sulfuric acid: For example, sodium is highly active and thus able to replace hydrogen from water:Ģ Na (s) + 2 H 2O (l) → 2 NaOH (aq) + H 2 (g) In general, the more reactive a metal is: the more vigorously it. Here is a series of some of the most common metals, listed in descending order of reactivity.Ī metal can replace metals listed below it in the activity series, but not above. The reactivity series of metals is a chart listing metals in order of decreasing reactivity. This is markedly different from the table below. The reactivity series taught in the US is defined by the ease of oxidation and corresponds to the ordering of the table of standard electrode potentials. In the UK a reduced version of the series below is taught as part of the GCSE chemistry course, leading to various mnemonics being invented to aid memory.







 0 kommentar(er)
0 kommentar(er)
